Abstract
Background: While myeloma remains an incurable disease, novel therapies have significantly improved survival. Daratumumab is a human IgG1κ anti-CD-38 monoclonal antibody that is approved in several different combinations in the first line and relapsed refractory setting (Daratumumab. Package insert. Janssen Biotech, Inc.; 2021.). However, there is little guidance on treatment selection and sequencing. Clinical pathways have been implemented at different institutions as a decision support tool for the treatment of complex diseases. Dana-Farber Cancer Institute (DFCI) Clinical Pathways includes daratumumab based regimens in 11 of 15 nodes for myeloma treatment.
Methods: This was a real-world, single-center, retrospective, observational study assessing daratumumab based navigations in multiple myeloma from August 2019 to August 2020. The primary objectives were to describe the usage and efficacy of daratumumab, and to evaluate the concordance between myeloma pathway navigation and actual clinical practice. We extracted all myeloma pathways navigations over a 13 month period, with specific focus on navigations for daratumumab-based regimens; for those patients, we performed chart review to determine efficacy endpoints (response rate, overall survival, time to response, duration of response, and time to treatment failure) and to compare the navigated pathway to the actual clinical setting and treatment. Differences between navigated clinical characteristics (e.g., line of therapy) or treatment selected compared to actual clinical context and/or treatment would be considered a discordant navigation.
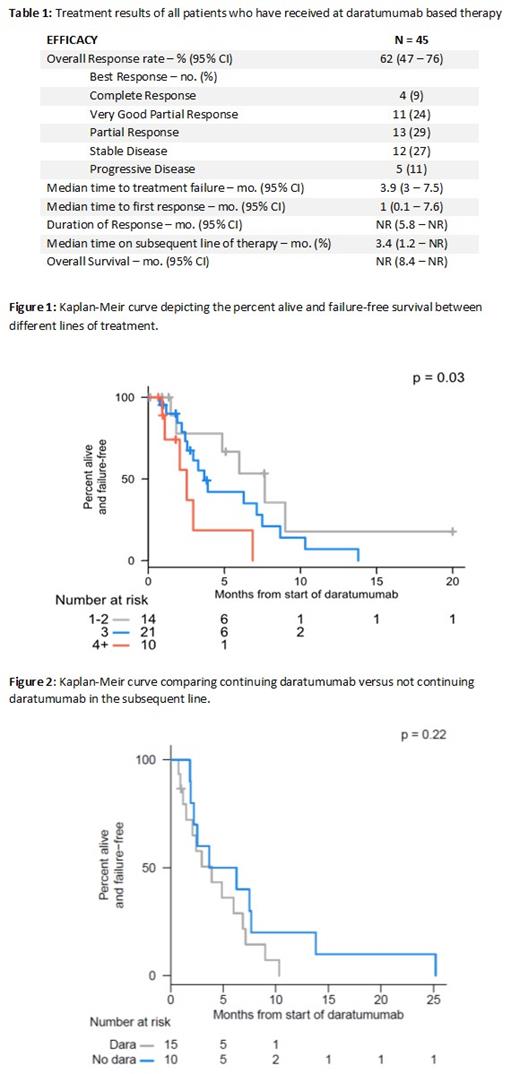
Results: There were 47 patients with a daratumumab based regimen, of which 45 patients received at least one dose of treatment. Daratumumab/pomalidomide/dexamethasone was used in 12 (45%) of patients, followed by daratumumab/bortezomib/dexamethasone 12 (26%), and daratumumab/dexamethasone in 6 (13%). Daratumumab/carfilzomib/lenalidomide/dexamethasone was used in 1 (2%) patient, and daratumumab/pomalidomide/bortezomib was used in 3 (6%) patients. Daratumumab based regimens were implemented as first line treatment in 1 (2%) patients, second line in 14 (30%) patients, third line in 21 (45%) patients, and fourth line or beyond in 11 (23%) patients. The overall response was 62% (95% CI: 47 - 76). Overall survival was not reached. The median time to first response and duration of response was 1 month and not reached, respectively (Table 1). The median time to treatment failure when daratumumab was used in the first line was not reached, 7.7 months in the second line, 3.7 months in the third line, and 2.5 months in the fourth line or beyond (Figure 1). Twenty-five (53%) patients moved on to subsequent therapy. This consisted of 12 (26%) patients adding a new medication to their current regimen, 3 (6%) patients continued daratumumab but changed combination agents to new treatments, and 10 (21%) patients receiving an entirely new regimen. There was no statistically significant difference in continuing daratumumab in the subsequent line (Figure 2). The median time on subsequent therapy was 3.4 months. Of the 47 navigations, 15 (32%) showed discordance between navigation and the actual clinical context. Reasons for discordance include discordance in the line of therapy (14.9%), drug selection in treatment plan (6.4%), both discordance in line of therapy and drug selection (4.3%), decision point discordance (4.3%) and no daratumumab received (2.1%).
Conclusion: This hypothesis generating study suggests that daratumumab may improve clinical outcomes in earlier lines of treatment with diminished returns in later lines. Daratumumab used in the second line improved time to treatment failure by 4 and 5.2 months when compared to the 3rd and 4th lines, respectively. In subsequent lines, continuing daratumumab did not confer any additional benefit.
While all patients received treatment as indicated by the medical chart, discordance between pathway navigation and clinical context occurred in 32% of patients. Reasons include differing definitions of line of therapy, select regimens only available in early lines of treatment, as well as the use of off-label combinations. The nuances of myeloma care present a challenge and opportunity for the development of clinical pathways and guidance in this field.
Mo: Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Epizyme: Consultancy; Eli Lilly: Consultancy; BMS: Membership on an entity's Board of Directors or advisory committees. Richardson: AstraZeneca: Consultancy; Karyopharm: Consultancy, Research Funding; Regeneron: Consultancy; Sanofi: Consultancy; Secura Bio: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Research Funding; Protocol Intelligence: Consultancy; GlaxoSmithKline: Consultancy; AbbVie: Consultancy; Oncopeptides: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding. Nadeem: Takeda: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal